Drugmaker Biogen has till 2030 to finish a research confirming whether or not its new drug Aduhelm actually slows the brain-destroying illness. That is beneath the phrases of the Meals and Drug Administration’s conditional approval of the drug, a call that has been praised by sufferers as overdue and condemned by the company’s personal exterior consultants.
However each camps agree: 2030 is much too lengthy to attend for solutions on the $56,000-a-year drug.
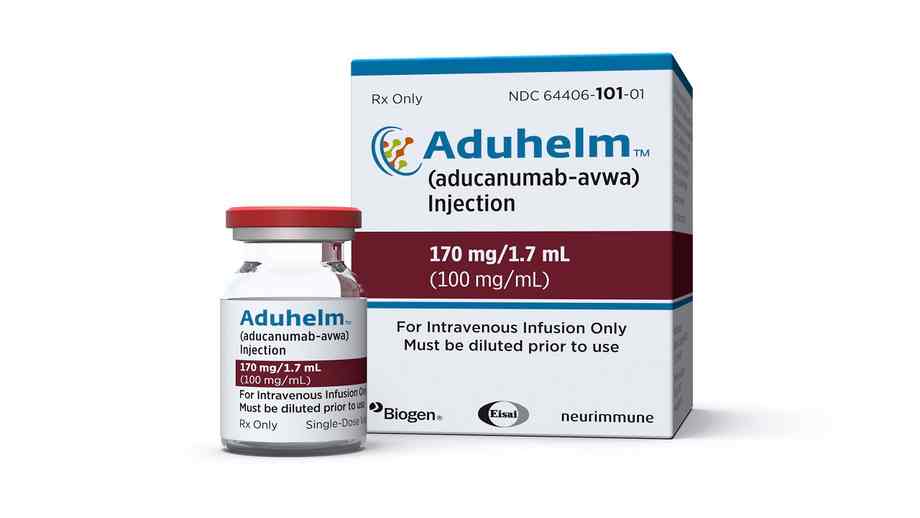
FILE – This picture supplied by Biogen on Monday, June 7, 2021 reveals a vial and packaging for the drug Aduhelm. (Biogen through AP, File)
“We expect 9 years is unacceptable and our expectation is that it’s going to occur in a a lot shorter time-frame,” mentioned Maria Carrillo of the Alzheimer’s Affiliation, an advocacy group that pushed for approval however now needs the FDA to set a faster deadline.
Different consultants warn that the 2030 timeline might slip if sufferers balk at enrolling in a brand new research for a drug that’s already accessible. And the give attention to Aduhelm — the primary new Alzheimer’s drug in 18 years — might steer volunteers away from testing of different promising therapies.
“If somebody can go to their doctor and get the FDA-approved drug, why would they go right into a trial the place they danger getting a placebo?” mentioned Donna Wilcock, an Alzheimer’s researcher on the College of Kentucky.
ALZHEIMER’S DRUG STIRS HOPE FOR PATIENTS, WORRY FOR DOCTORS
To determine a brand new drug’s security and effectiveness, researchers examine ends in individuals who get the remedy to an identical group of people that do not. That typically means half of the volunteers are randomly assigned to get a pretend remedy as a substitute of the true factor.
Biogen already carried out two such massive research of its drug, which requires month-to-month IVs. The research took about 4 years to run and adopted members for about 1 1/2 years. Each had been stopped early when it appeared the drug wasn’t working, and the outcomes had been so mired by flaws and inconsistencies that the FDA deemed them too weak to assist approval on the idea of slowing the illness.
As a substitute, the company took one other method and gave the drug conditional approval primarily based on a promising signal: its success in eliminating a buildup of sticky plaque within the mind that’s thought to play a job in Alzheimer’s illness.
FDA ADVISER WHO RESIGNED OVER ALZHEIMER’S DRUG SAYS ‘NO GOOD EVIDENCE’ IT WORKS
Beneath its so-called accelerated approval program, the FDA is requiring Biogen to conduct a brand new research definitively answering whether or not Aduhelm’s impact on plaque actually slows psychological decline in sufferers. Different Alzheimer’s medicine available on the market solely quickly ease signs.
The FDA has not detailed how the 2030 goal took place, or why such a distant deadline was granted for a drug that might be given to tens of millions of sufferers within the coming years, including billions to the nation’s well being care invoice.
“Alzheimer’s trials take time to finish,” the FDA mentioned in an announcement responding to questions concerning the research. The company added that it may be attainable to reply key questions on Aduhelm earlier than the research’s completion and that Biogen is anticipated to submit outcomes “as quickly as possible.”
BIOGEN’S DEBATED ALZHEIMER’S DRUG GIVEN TO FIRST PATIENT
However the company’s critics level out that 9 years is among the many longest follow-up intervals the company grants drugmakers. Medicine permitted beneath related circumstances sometimes get six years. And, if something, these research are likely to run not on time, not end early. If follow-up research haven’t got optimistic outcomes, the FDA can withdraw approval, although it not often does so.
“Simply because it says 9 years doesn’t imply the proof will likely be accessible in 9 years,” mentioned Joshua Wallach, a medical researcher at Yale’s Faculty of Public Well being. “There’s all of this backwards and forwards dialogue that may occur with FDA that may delay completion.”
Biogen isn’t scheduled to submit its preliminary proposal for the research to FDA till October. The Massachusetts-based firm mentioned in an announcement that enormous Alzheimer’s trials usually take six or seven years and that FDA-mandated research can take even longer.
2 FDA COMMITTEE MEMBERS RESIGN OVER BIOGEN ALZHEIMER’S DRUG APPROVAL
“We’re working with urgency and placing sources and plans in place,” to finish the trial forward of schedule, the corporate acknowledged.
In the meantime, Alzheimer’s specialists like Dr. Samuel Gandy are seeing sufferers in different drug research ask about dropping out to allow them to get Aduhelm.
“They’ve all mentioned, ‘, I can’t stand the thought of being on placebo,’” mentioned Gandy, who has heard from greater than 20 households within the drug at New York’s Mount Sinai hospital.
WHO SHOULD TAKE NEW ALZHEIMER’S DRUG? EXPERT WEIGHS IN
After he defined the drug’s unknown advantages and potential unwanted side effects — together with mind swelling and bleeding — a number of determined in opposition to it. However different sufferers stay .
Put up-approval research have develop into an more and more frequent FDA requirement because the Nineties, as regulators have accelerated their opinions of medication for HIV, most cancers and different lethal illnesses. However the company’s combined report of monitoring these necessities and penalizing firms that don’t meet them has been chronicled in authorities and educational research.
The case of a extensively debated drug for muscular dystrophy illustrates how the system can go awry.
FDA OK’S BIOGEN’S ALZHEIMER’S DRUG: WHAT IS ACCELERATED APPROVAL?
In 2016, the FDA permitted the first-of-a-kind drug from Sarepta Therapeutics primarily based on preliminary outcomes that it would assist deal with the degenerative illness by boosting a muscle-building protein.
As with Aduhelm, the approval was opposed by FDA’s exterior advisers who mentioned there was scant proof the drug truly improved affected person well being or high quality of life. However the FDA granted approval on the situation that Sarepta full a confirmatory research by Might 2021.
The trial, although, remains to be getting underway after “a number of challenges within the general planning and startup,” based on the FDA’s web site. The brand new goal date is 2026, a decade after the drug was allowed available on the market.
FDA APPROVAL OF BIOGEN’S ALZHEIMER’S DRUG LEAVES SOME ‘DISAPPOINTED’
A Sarepta spokeswoman mentioned the corporate spent years negotiating research particulars with the FDA, which required testing a better dose.
Within the meantime, Sarepta has received approval for 2 different dystrophy medicine primarily based on related outcomes that additionally require follow-up trials, which the corporate says are already nicely underway.
“The FDA took a danger with Sarepta and I believe they’re being burned by it now,” mentioned Dr. Joseph Ross of Yale.
Ross and his colleagues have proven that at the very least 1 / 4 of follow-up outcomes by no means get printed, leaving questions for physicians and sufferers.
The outcomes from Biogen’s two Aduhelm research have but to seem in a medical journal. The corporate says it’s “working diligently to publish our information.”