CLICK HERE TO FIND A COVID-19 VACCINE NEAR YOU
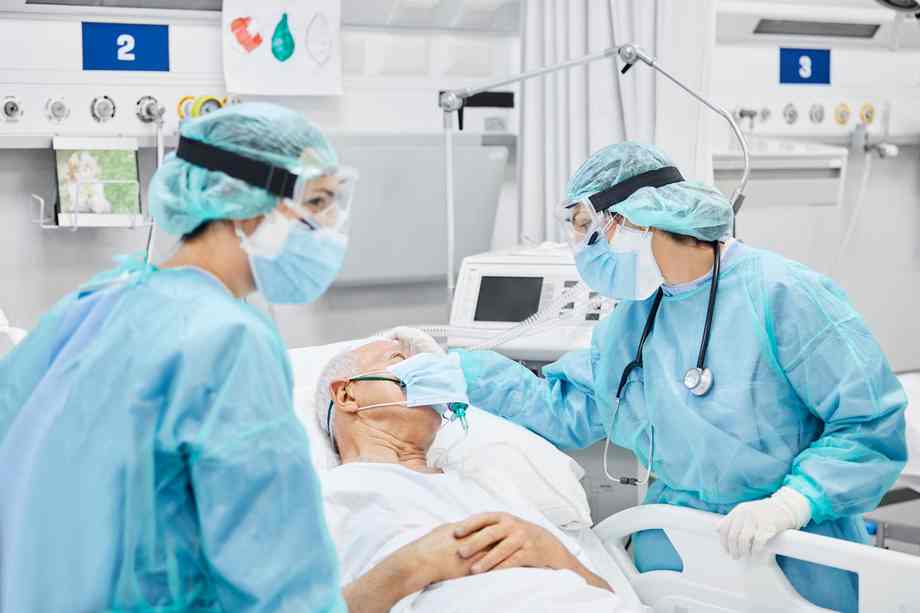
The research, which concerned a cohort of 101 grownup sufferers, discovered these on mechanical air flow or extracorporeal membrane oxygenation (ECMO) who got the drug and customary of care had been 46% much less more likely to die by day 28 in comparison with those that obtained a placebo and customary of care.
Within the COV-BARRIER trial, 58% of sufferers within the placebo arm died by day 28, in comparison with 39.2% within the baricitinib group. By day 60, the proportion rose to 62% among the many placebo arm, in comparison with 45.1% within the baricitinib group. No new security alerts had been recognized in both group.
FLORIDA HOSPITALS FACING RECORD COVID-19 SURGE EXPAND UNITS, LIMIT VISITORS AGAIN
The research has not but undergone peer evaluation, though the corporate stated it intends to submit it for publication within the coming months.
“As further knowledge from COV-BARRIER change into obtainable, it’s more and more evident that therapy with baricitinib could assist stop loss of life in among the most critically in poor health COVID-19 sufferers and that baricitinib represents an vital therapy choice for this weak group of sufferers on this consistently evolving pandemic,” E. Wesley Ely, MD, MPH, professor of medication and co-director of essential sickness, mind dysfunction and survivorship heart at Vanderbilt College Medical Middle, stated, in a information launch.
The FDA moved final week to increase emergency use authorization to permit baricitinib to be given with or with out remdesivir. The drug can now be given to hospitalized adults and pediatric sufferers ages 2 and older who require supplemental oxygen or air flow.
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
The drug is a once-daily oral JAK inhibitor and has been used to deal with adults with moderate-to-severe rheumatoid arthritis.